Radii Trends
Build atoms of increasing atomic radius by adding protons to the nucleus, and electrons to the atomic orbitals while discovering fill order.
Topics
- Atomic Neutrality
- Pauli Exclusion Principle
- Aufbau Principle
- Hund's Rule
- Atomic Radii Trends
- d-orbitals
- Electronegativity
- Valence electrons
Take a peek inside the Radii Trends game for a brief overview of the concepts covered through gameplay. Learn about the challenge levels, exploratory sandbox, and free teacher resources for use in your chemistry classroom.
Core levels
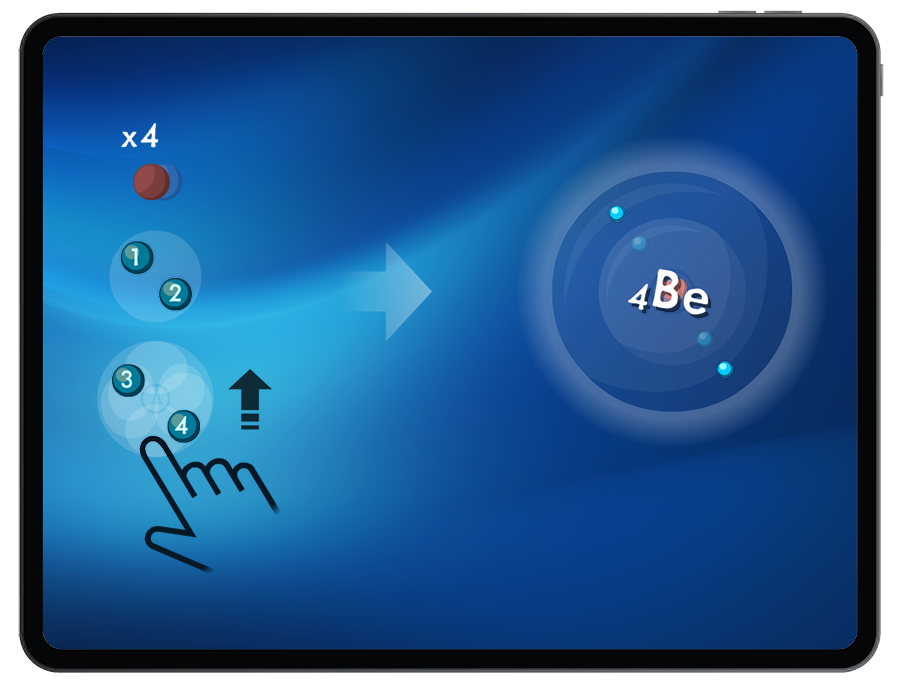
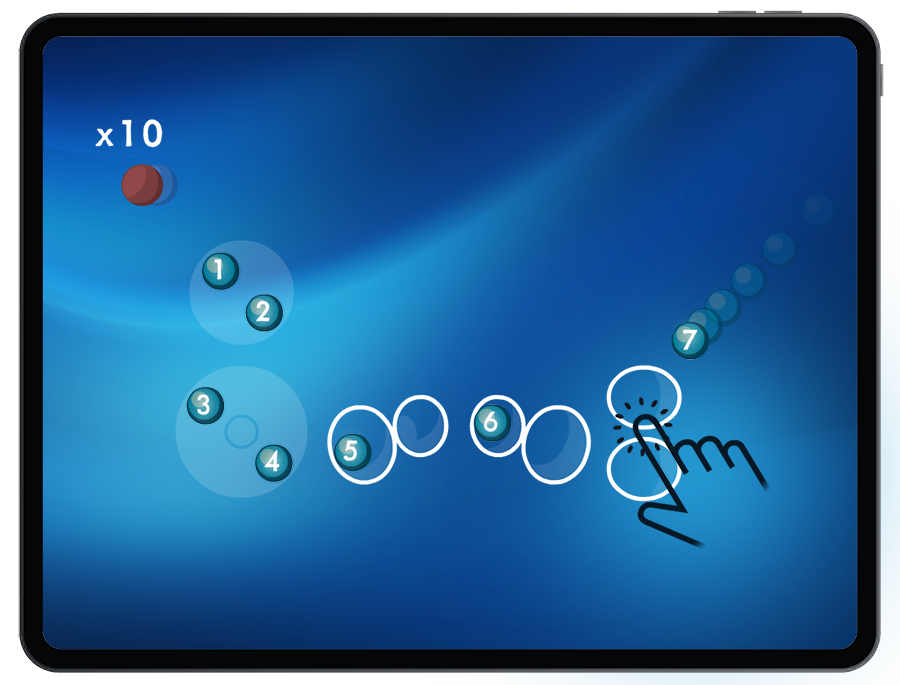
To successfully build atoms, students must add an equal number of protons and electrons.
When adding electrons to the orbitals, Students explore the patterns of electron fill order (Pauli Exclusion Principle, Hund’s Rule and Aufbau Principle) and electron configuration.
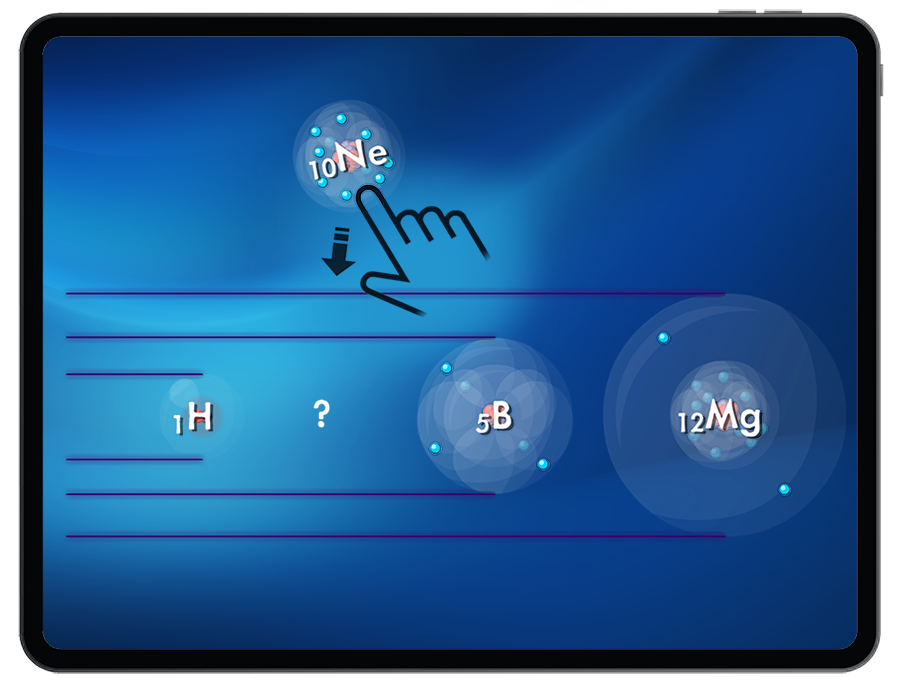
Students are introduced to the periodic trend of atomic radii by being challenged to create atoms of increasing size.
Sandbox
The Radii Trends Sandbox provides an open-ended and exploratory environment for students to freely build and compare atomic radii of atoms. Design your own extension activities using the Sandbox, and encourage your students to earn the built-in Achievements that focus on a specific topic.