Lewis Structures
Bond together atoms to create target molecules with unique bond polarities and molecular shapes.
Topics
- Octet/duet rule
- Single bonds
- Double and triple bonds
- Non-bonded domains
- Electronegativity
- Bond polarity
- Number of electron domains
- Molecular shape/VSEPR
Take a peek inside the Lewis Structures game for a brief overview of the concepts covered through gameplay. Learn about the challenge levels, exploratory sandbox, and free teacher resources for use in your chemistry classroom.
Core levels
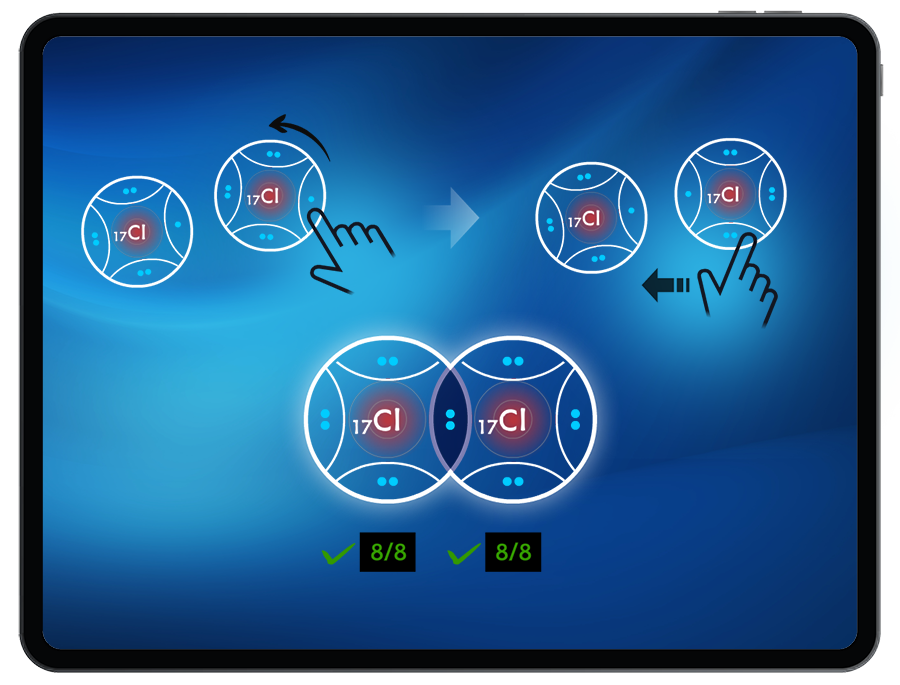
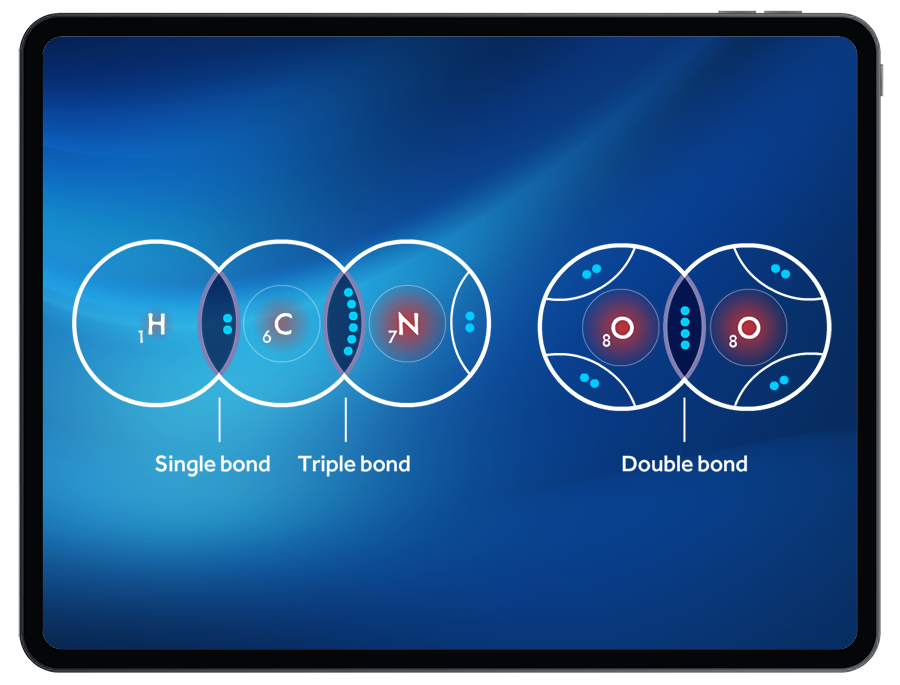
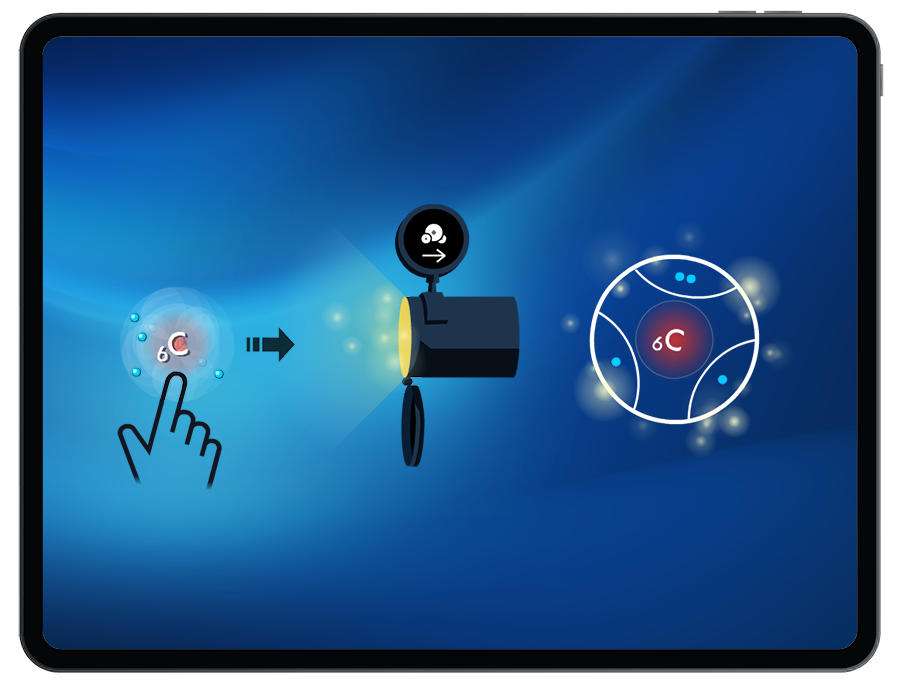
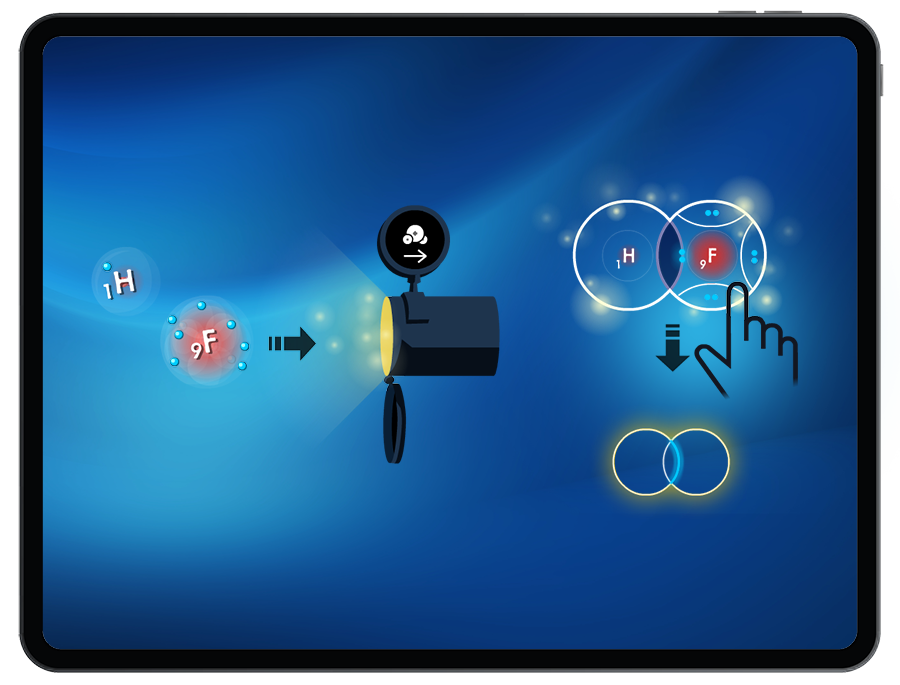
To successfully create a molecule, students must satisfy the octet (or duet) rule when bonding atoms together. By manipulating the number of shared valence electrons between atoms, students can create single, double, and triple bonds.
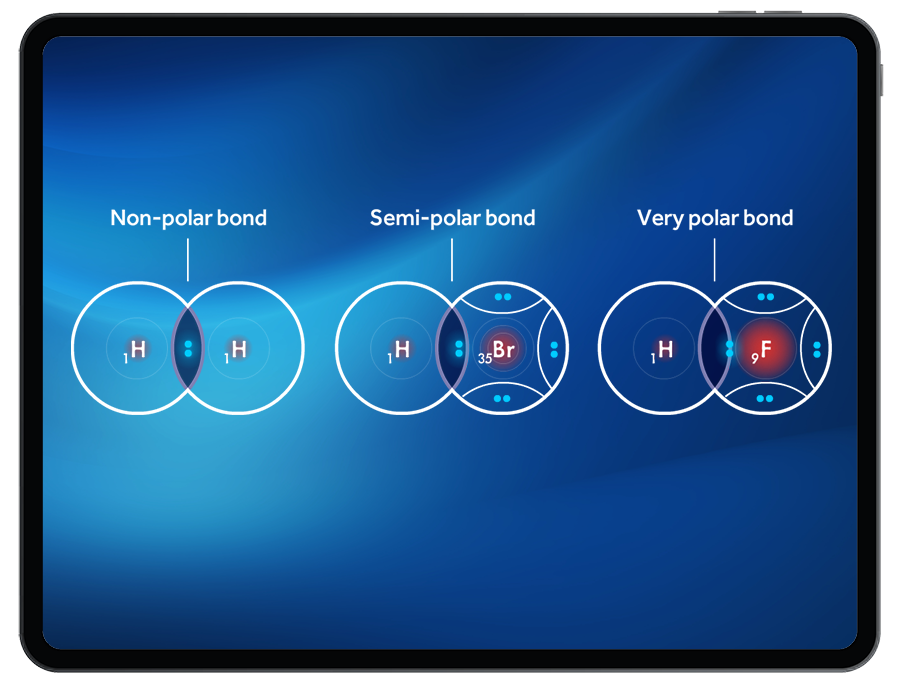
Through observing the location of the shared electrons in the bonds created, and the relative electronegativity of the atoms, students will explore non-polar, semi-polar, and very polar covalent bonds.
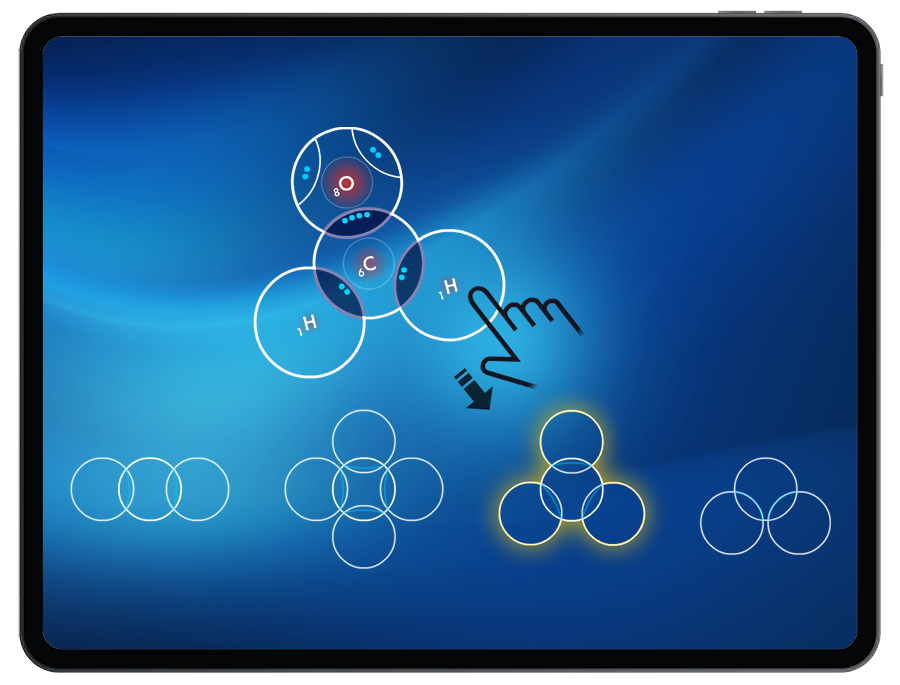
Students will be introduced to VSEPR Theory by building molecules that match the molecular shapes presented in the targets (linear, bent, trigonal planar, tetrahedral).
Sandbox
Connected levels
Radii Trends and Lewis Structures
In these connected game levels, there are atoms missing from the provided bank. Students must return to the Radii Trends game to build the missing atoms with the correct number of valence electrons and electronegativity level that they need in order to create the target molecules in the Lewis Structures game.