LeChâtelier
Disturb reactions to shift equilibrium and cause changes in the system to match game targets.
Topics
- Reversible reactions
- Relative reaction rates: forward versus reverse
- Relative Kc
- LeChâtelier’s Principle: effects of changes in
- Concentration
- Temperature
- Pressure
Core levels
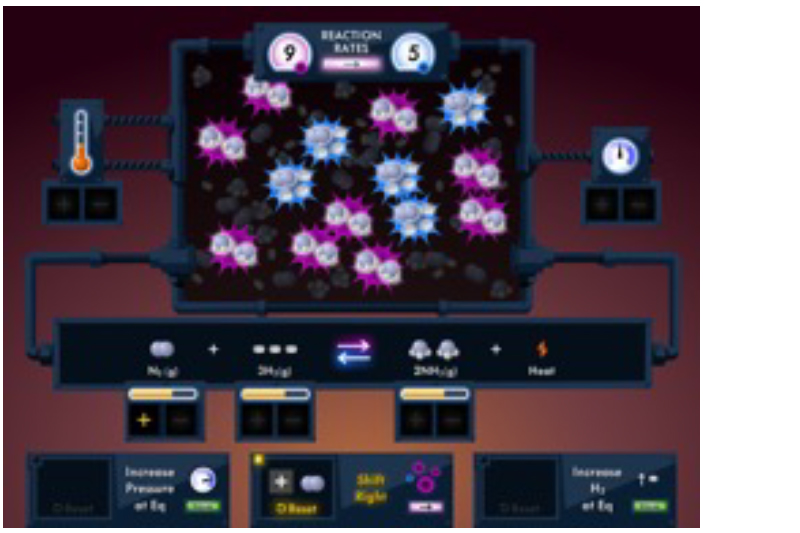
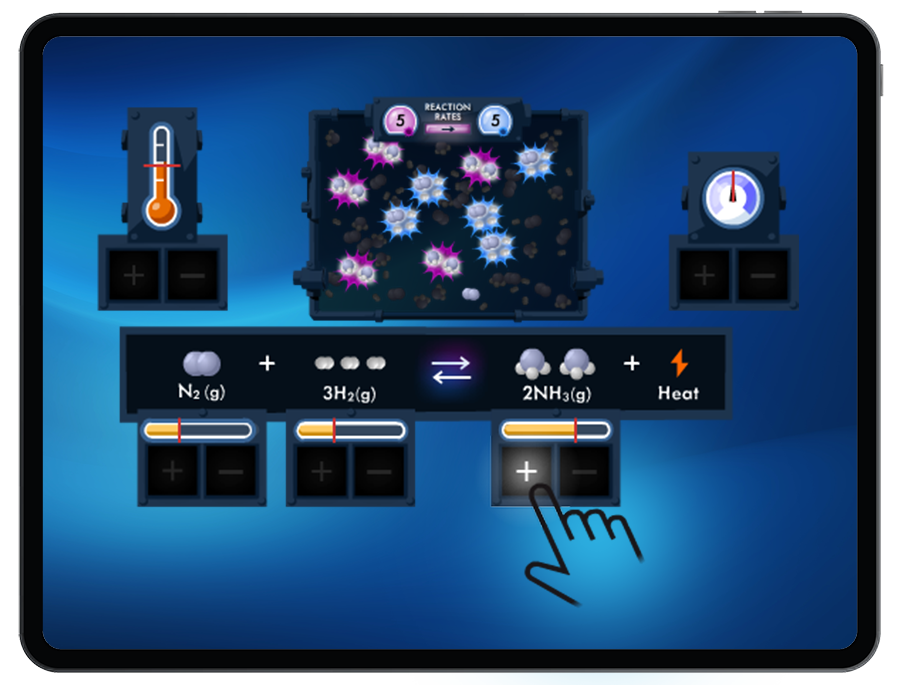
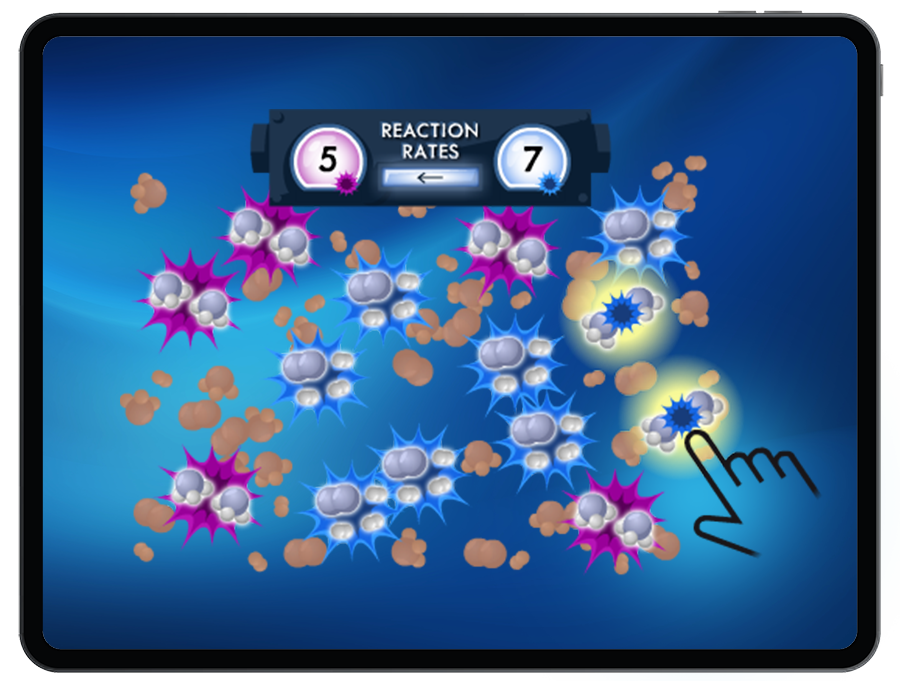
Students are presented reversible reactions at equilibrium, including gaseous systems and aqueous solutions. Students then disturb the equilibrium by changing the concentration of a substance, the temperature, or the pressure in order to shift equilibrium as governed by LeChatelier’s Principle.
Students will observe the system return to equilibrium with resulting changes in reactant concentrations, product concentrations, temperature and pressure.
Sandbox
The Sandbox provides an open-ended and exploratory environment for students to freely select a reaction, disturb it, and shift equilibrium. Design your own extension activities using the Sandbox and encourage your students to earn the built-in Achievements that focus on a specific topic within Equilibrium (i.e., the Haber process, dissociation of an ionic solid, changing the container size).