Latent Heat
Match the sequence of phases in each target by adding or removing energy to affect particle motion and IMFs.
Topics
- Melting/freezing point
- Boiling/condensation point
- Sublimation/deposition
- Relative kinetic energy of phases
- Intermolecular forces
- Endothermic versus exothermic processes
- Potential versus kinetic energy
- IMF strengths and boiling points
- IMF vs ionic bonding
Core levels
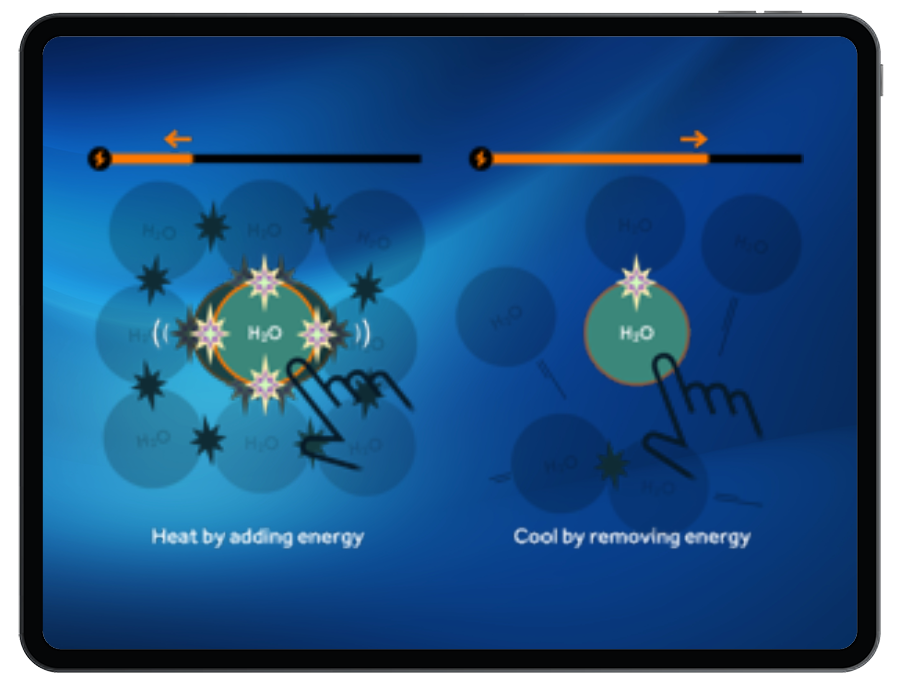
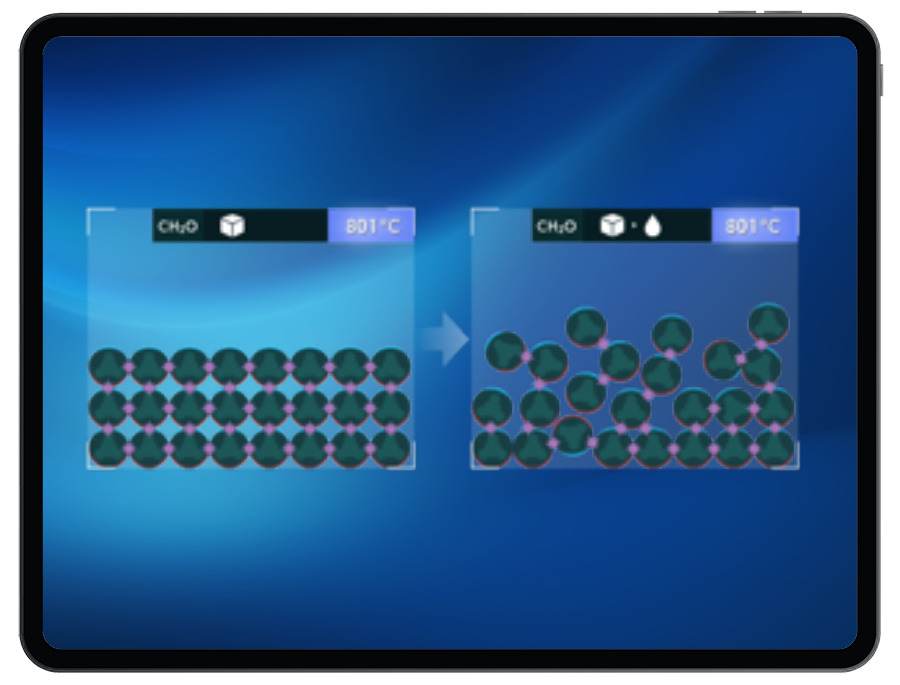
Students must heat or cool particles by adding or removing kinetic energy to complete the phase change of the targets.
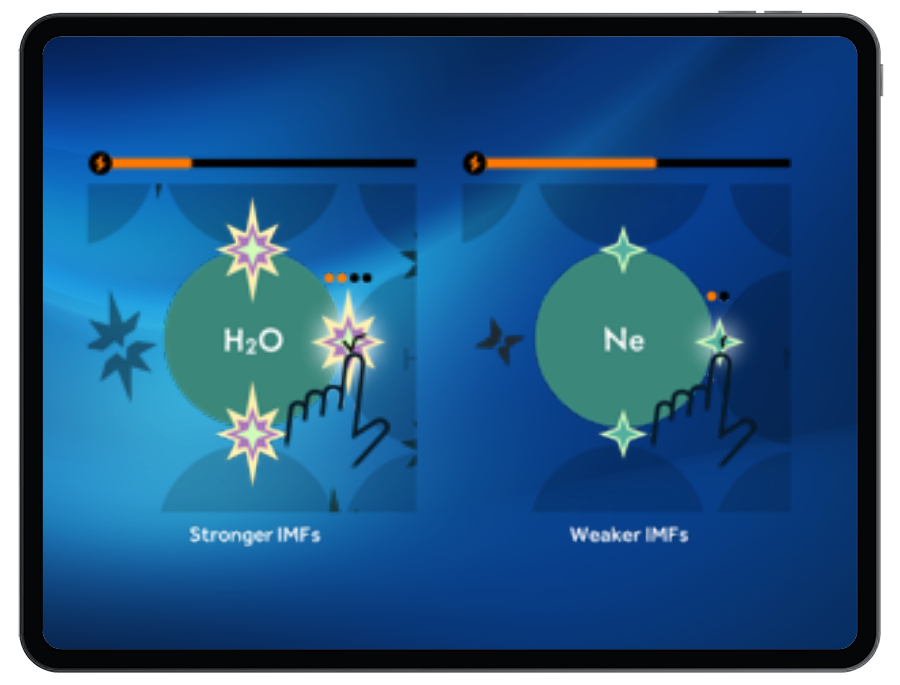
Students will break or form IMFs between particles during a phase change. Stronger IMFs require more energy to break, and release energy when formed.
Students will observe that phase changes occur at a constant temperature due to latent heat.
Sandbox
The Sandbox is an open-ended and exploratory environment designed for students to freely explore phase change using the provided bank of particles. Complement your instruction by designing your own Sandbox activities and encourage your students to earn the built-in Achievements that focus on a specific topic within Latent Heat.
Connected levels
IMFs & Latent Heat
In these connected levels, there are molecules missing from the bank. Students must return to the IMFs game to build molecules that have IMFs that specifically allow for the phase change needed to correctly match the targets. Students are prompted to think about how IMF strength affects the amount of energy released or absorbed during the breaking and forming of IMFs during phase change. Stronger IMFs release more energy when forming.
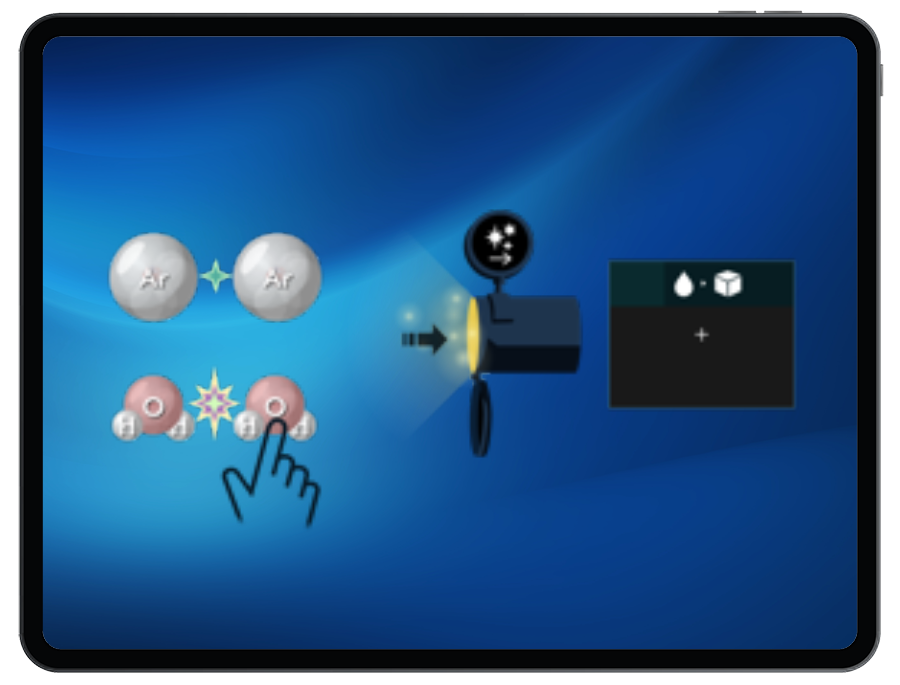
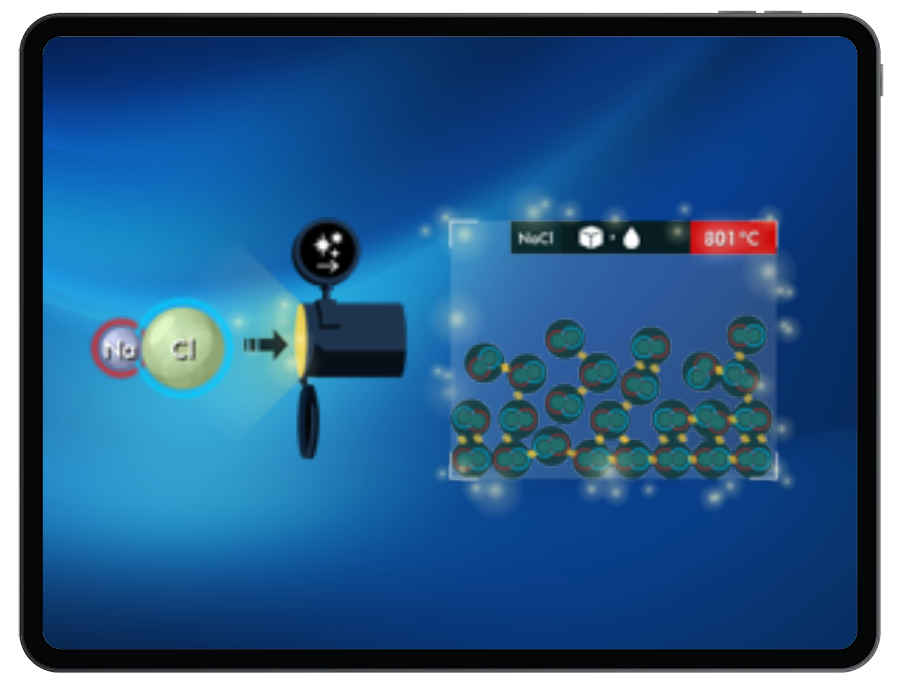
Ionic Bonding & Latent Heat
In these connected levels, there are ionic compounds missing from the bank. Students must return to the Ionic Bonding game to build ionic compounds that can be used in the Latent Heat game given the energy provided. Once built, Students can send these compounds through the pipe, heat or cool the compound, and break or form ionic bonds to change phases and satisfy the target.