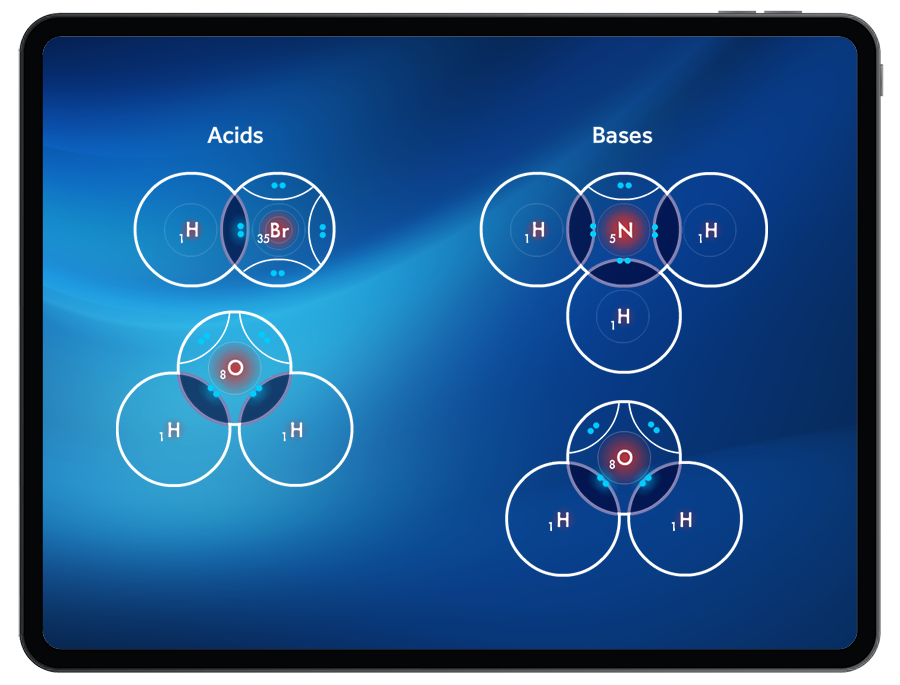
Acid Strength
Remove protons from acids and add protons to bases to create target ions and molecules.
Topics
- Brønsted-Lowry acids and bases
- Electronegativity differences
- Strong versus weak acids
- Percent dissociation
- Neutralization reactions
- Polyprotic acids
- Amphoteric substances
- Conjugate acids and conjugate bases
- Charge of resulting ions
Core levels
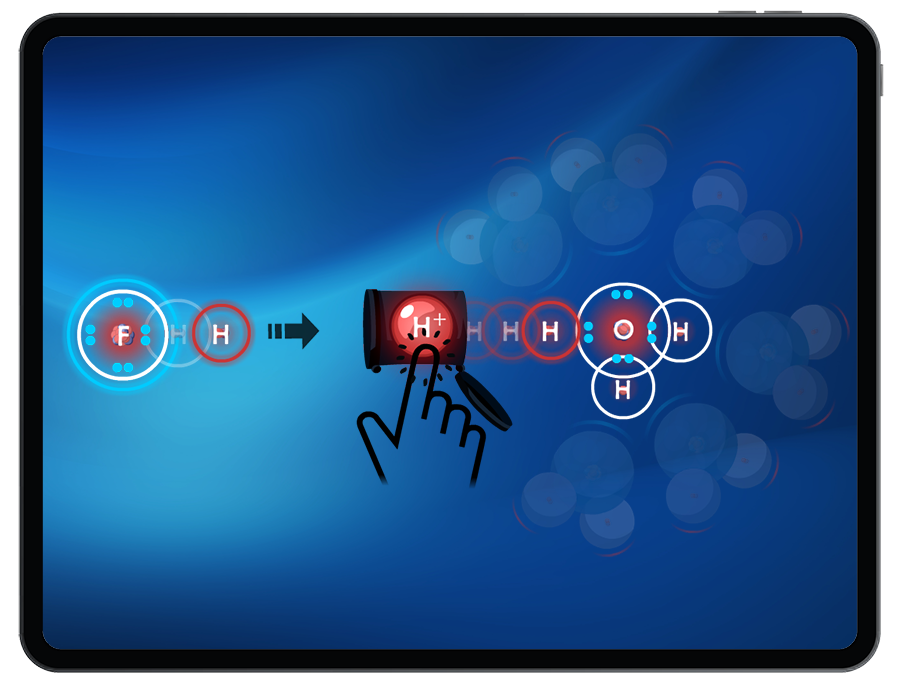
Through observing acids as proton donors and bases as proton acceptors, Students are introduced to the Bronsted-Lowry definition of acids and bases.
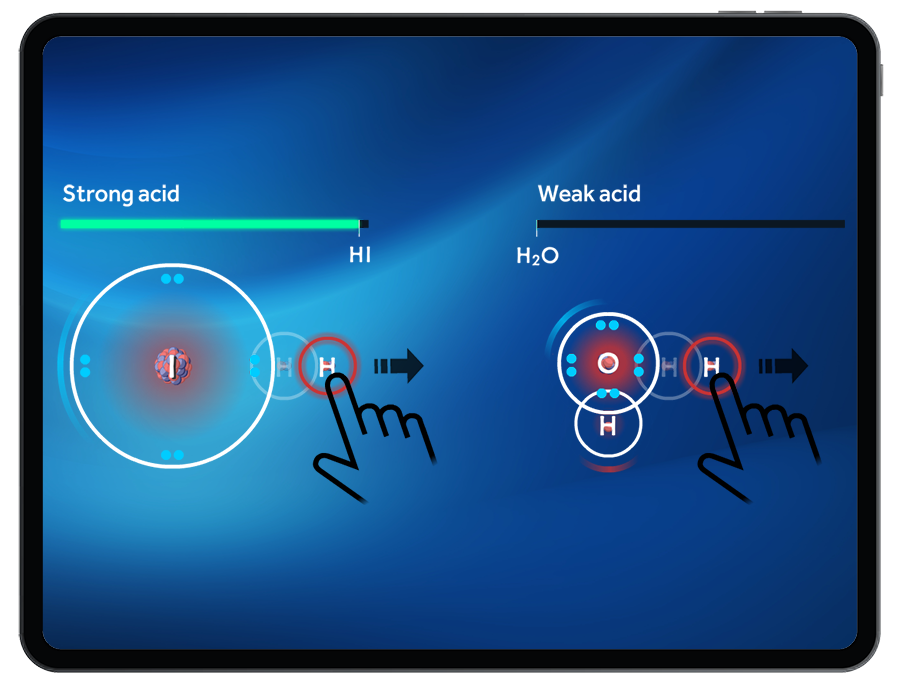
Students can compare strong vs. weak acids by exploring the ‘ease’ of removing a proton. It will be easier to remove a proton from a strong acid than from a weak acid.
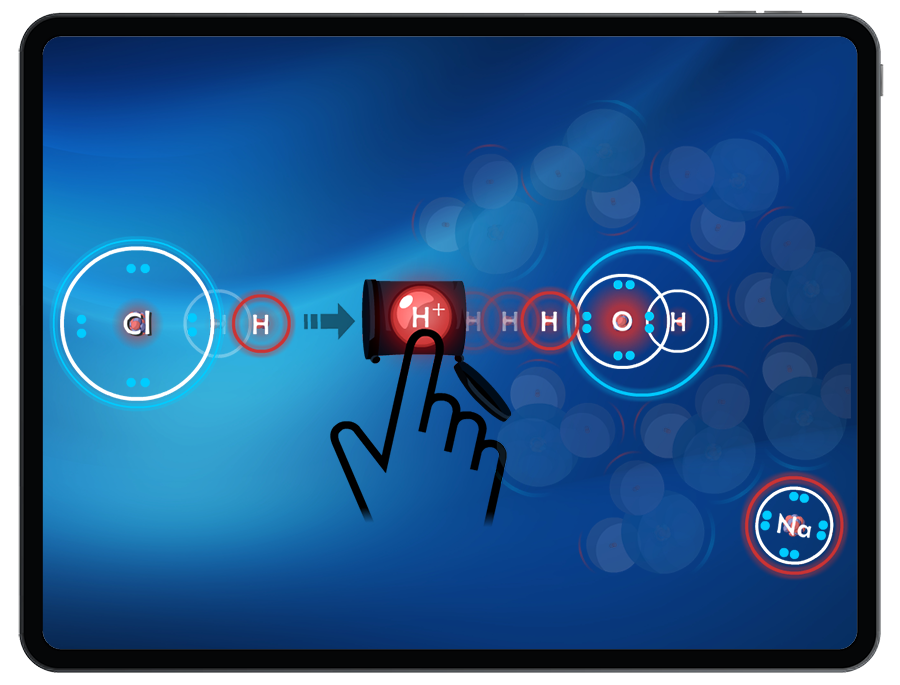
In several levels, students will conduct neutralization reactions to create water and various salt compounds.
Sandbox
The Sandbox is an open-ended and exploratory environment designed for students to freely explore acids and bases. Complement your instruction by designing your own Sandbox activities and encourage your students to earn the built-in Achievements that focus on a specific topic within Acid Strength (i.e. acid strength, neutralization, polyprotic acids, amphoteric substances).
Connected levels
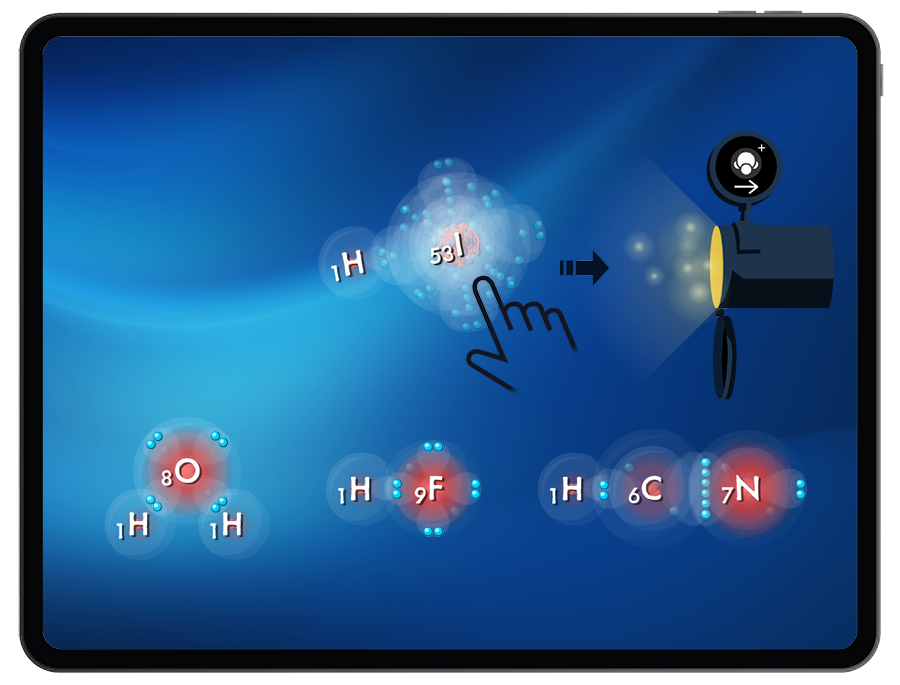
Lewis Structures and Acid Strength
In these connected levels, there are molecules missing from the bank. Students must return to the Lewis Structures game to build missing molecules that are strong enough acids to be protonated with the available energy in the Acid Strength game. These molecules can then be sent through the pipe to be used in the Acid Strength game.