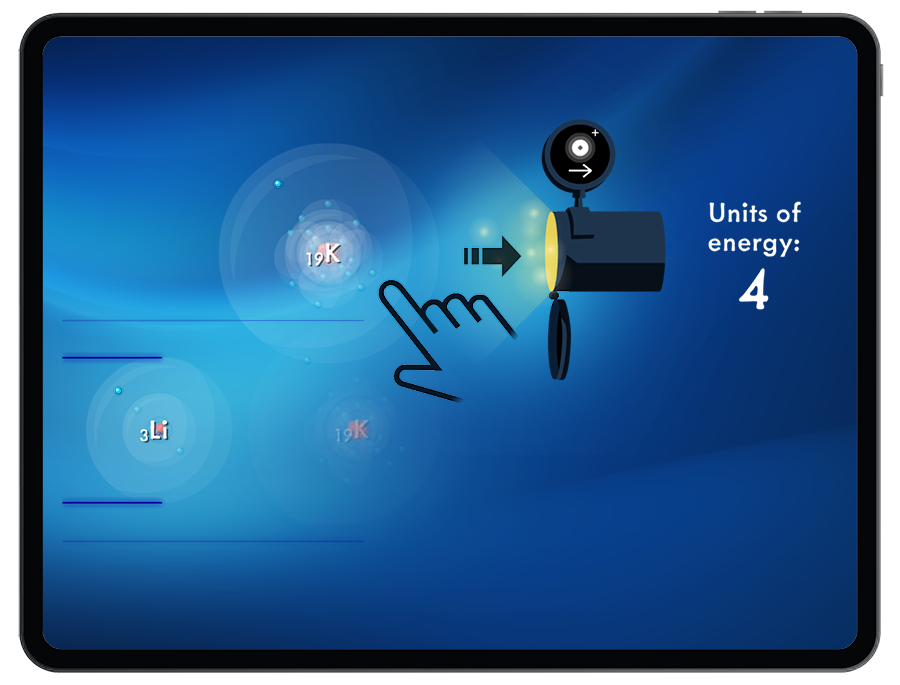
Ionization Energy
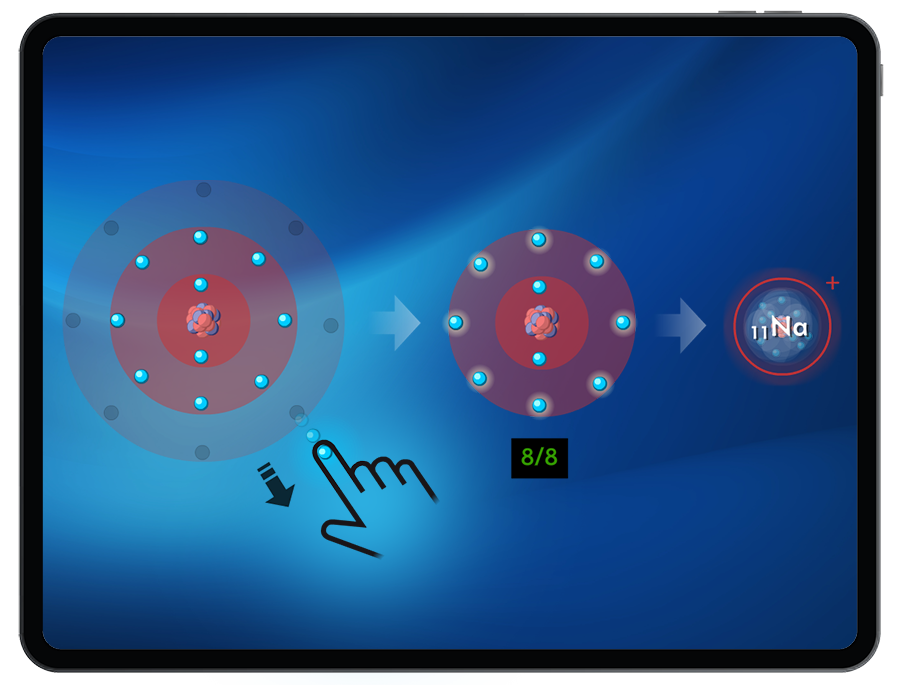
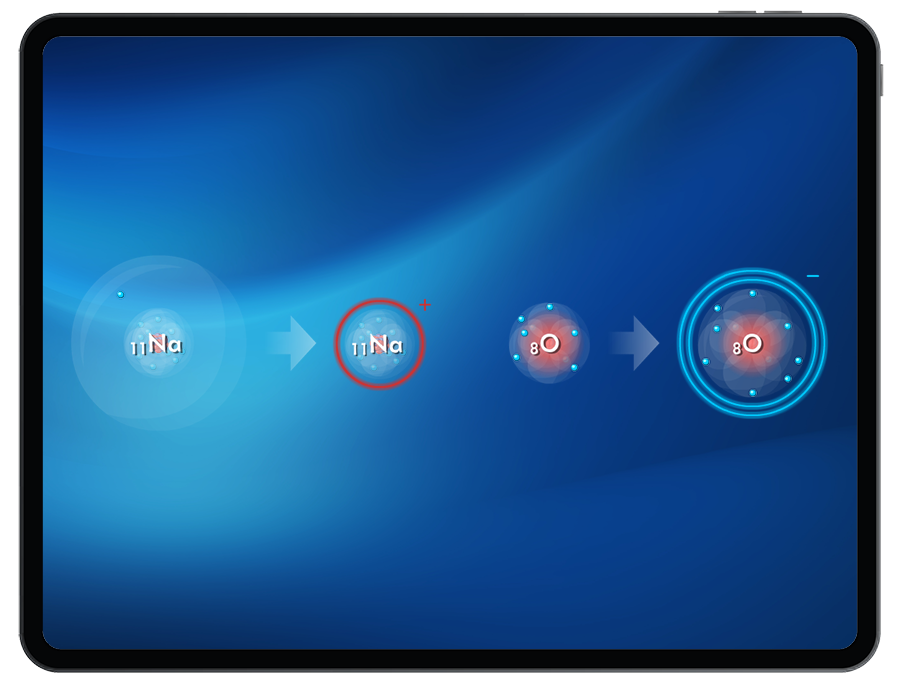
Form stable ions by attempting to add or remove electrons while monitoring available energy in order to create target cations and anions.
Topics
- Cation formation
- Anion formation
- Octet rule
- Valence electrons/ion charge
- Ionization energy trends
- Electron affinity trends
- Ionic radii trends
Take a peek inside the Ionization Energy game for a brief overview of the concepts covered through gameplay. Learn about the challenge levels, exploratory sandbox, and free teacher resources for use in your chemistry class.
Core levels
Students must add or remove the correct number of electrons from an atom to create an ion that satisfies the octet rule.
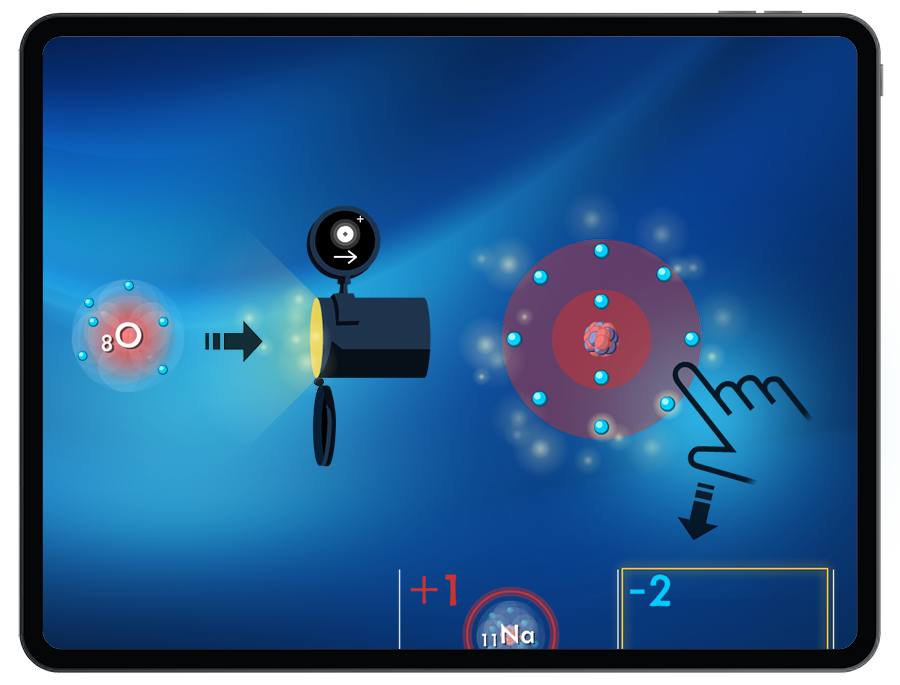
By exploring the energy required or released when adding and removing electrons, students are introduced to the periodic trends of ionization energy and electron affinity.
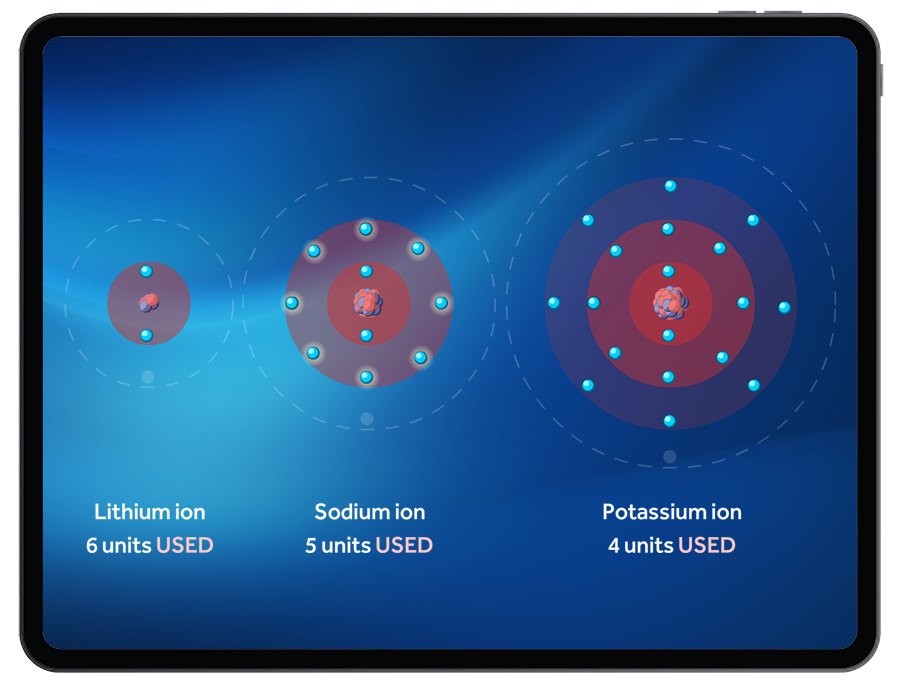
Students can observe the change in size between ions and their parent atoms, prompting the exploration of ionic radii.
Sandbox
The Ionization Energy Sandbox is an open-ended and exploratory environment designed for students to freely build ions using the provided bank of atoms. Complement your instruction by designing your own Sandbox activities and encourage your students to earn the built-in Achievements that focus on a specific topic.
Connected levels
Radii Trends and Ionization Energy
In these connected game levels, there are atoms missing from the Ionization Energy bank. Students must return to the Radii Trends game to build atoms that have the correct number of valence electrons needed to form the target ions. Once constructed, students can send these atoms through the pipe to ionize in the Ionization Energy game, within the limited amount of energy allotted.